
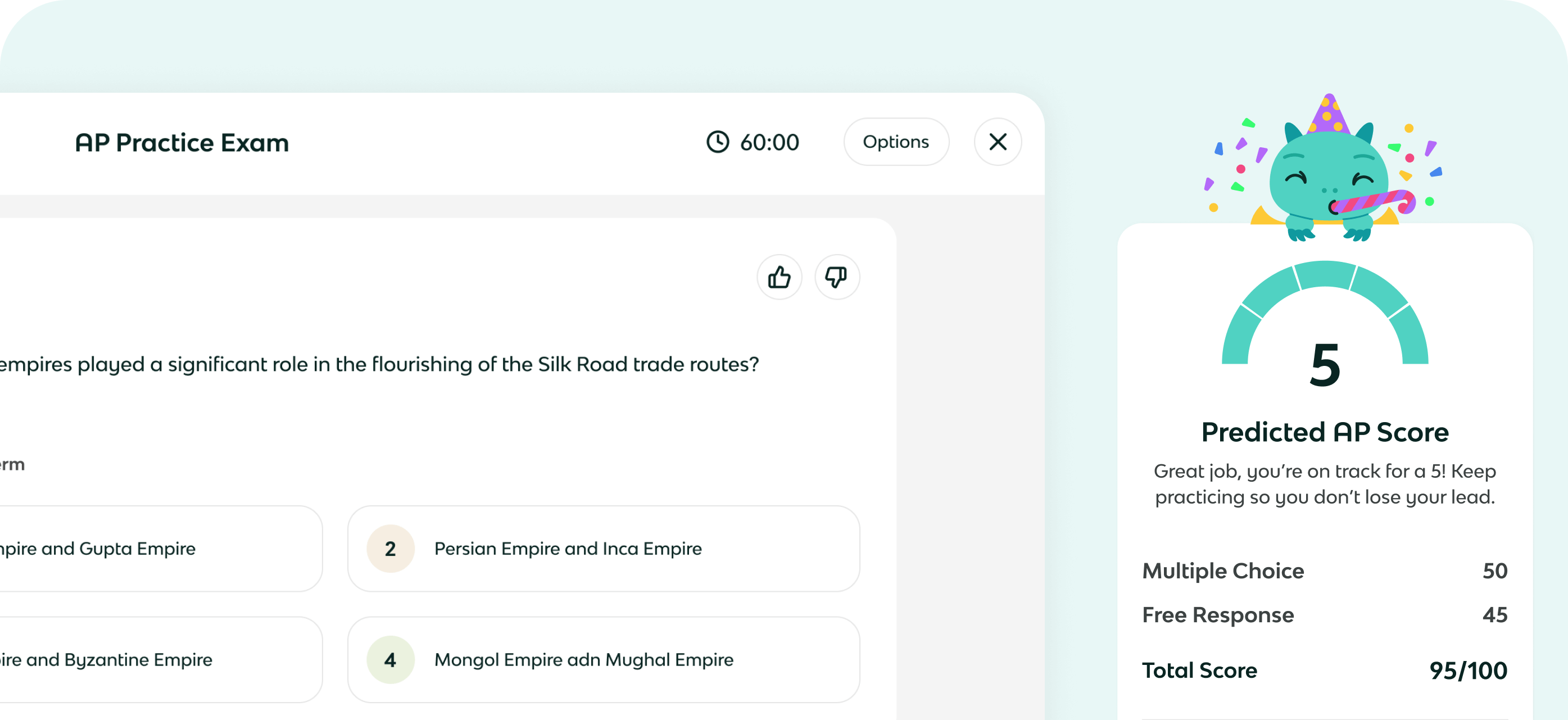
AP Chemistry FRQ Room
Ace the free response questions on your AP Chemistry exam with practice FRQs graded by Kai. Choose your subject below.
Knowt can make mistakes. Consider checking important information.
AP Chemistry Free Response Questions
The best way to get better at FRQs is practice. Browse through dozens of practice AP Chemistry FRQs to get ready for the big day.
Analyzing Electron Shielding Effect in Successive Elements
This question examines how electron shielding affects the effective nuclear charge, and how this in
Catalytic Reaction Rate Experiment with Temperature Control Error
A chemical kinetics experiment is carried out to study the effect of various catalysts on the rate o
Comparative Analysis of Photoelectron Spectra for Different Elements
Two photoelectron spectra are obtained for Elements A and B. The spectrum for Element A shows narrow
Comparing the Bohr Model and the Quantum Mechanical Model
Compare the Bohr Model and the Modern Quantum Mechanical Model in terms of how they describe electro
Computational Study on Effective Nuclear Charge and Atomic Radius
A computational simulation was performed to study the relationship between effective nuclear charge
Coulomb's Law and Atomic Structure
Coulomb's law is given by the equation $$F = k * \frac{q_1 * q_2}{r^2}$$. In atomic systems, this la
Coulomb's Law and Electron Binding Energy
Using Coulomb's Law, calculate the magnitude of the electrostatic force between a proton and an elec
Coulomb's Law and Electron Removal Energy
This question requires the application of Coulomb's Law to quantify the electrostatic force between
Decomposition Reaction and Gas Laws
Consider the thermal decomposition of potassium chlorate, \(KClO_3\), which produces oxygen gas. The
Determining the Empirical Formula of an Unknown Hydrocarbon Using Combustion Analysis
A sample of an unknown hydrocarbon is subjected to complete combustion, and you are provided with th
Electron Configuration and Orbital Filling
This question assesses your understanding of quantum mechanical principles as applied to electron co
Examining Electron Shielding and Its Effects on Ionization Energy
Discuss the role of electron shielding in determining ionization energy and atomic radius.
Identification of Unknown Element Using Mass Spectrometry
A researcher is given a mass spectrum of an unknown element. The spectrum shows a major peak at 27.0
Investigation of Reaction Mechanism and Rate Laws
A reaction following the overall stoichiometry $$A + B \rightarrow C$$ is observed to proceed via a
Ionization Energy Determination Experiment with Overlooked Electron Screening
In an experiment to measure the ionization energy of a multi-electron atom using photoelectron spect
Mass Spectrum Analysis of Selenium Isotopes
A mass spectrum for selenium shows data for its naturally occurring isotopes as given in the table.
Measuring Coulombic Forces in Atoms via Ionization Energy Measurements
Coulomb's law predicts that the force between charged particles depends on both the magnitudes of th
Molarity and Dilution Calculations in Solution Preparation
A researcher needs to prepare 250 mL of a 0.500 M sodium chloride (NaCl) solution from a stock solut
Percent Composition Calculation and Error Analysis
A compound of calcium nitrate, $$\text{Ca(NO}_3)_2$$, is analyzed to determine its percent compositi
Periodic Trends and Bond Formation
A researcher explores how periodic trends influence the type of bonds formed between elements. Focus
Periodic Trends: Atomic Radius and Ionization Energy
The table below provides data for atomic radii and first ionization energies for a series of element
Predicting Reaction Outcomes Using Periodic Trends
In an experiment, several metals (designated as A, B, and C) were reacted with dilute acid, and the
Relationship Between Bond Enthalpy and Electronegativity Differences
Bond enthalpy is influenced by the difference in electronegativity between the bonded atoms. Design
Solution Molarity Calculations
Relate moles, grams, and volume by addressing solution concentration.
Stoichiometry and Percent Yield Calculation
An experiment is conducted where aluminum reacts with chlorine gas to form aluminum chloride accordi
Titration Experiment for Unknown Acid Molarity Determination
Titration is a common method for determining the molarity of an unknown acid solution. Design an exp
Valence Electrons and Chemical Reactivity: A Comparative Study
This question compares chemical reactivity between elements by analyzing their electron configuratio
Advanced Ionic Compound Lattice Energy Analysis: A Comparative Study
In this question, you will derive the relationship between ionic charges, interionic distance, and l
Analysis of Ionic Lattice Structure
Examine the diagram provided for an ionic lattice. In an ionic compound, the stability arises from t
Application of Coulomb's Law in Ionic Bonding
Using Coulomb's law, analyze the factors that affect the strength of ionic bonds in solids. Answer t
Bond Formation and Stability: Investigating Covalent Networks
This question examines how the network covalent structure contributes to the thermal stability of co
Comparative Analysis of Bonding Types and Electrical Conductivity
The conductivity of substances in various phases gives insight into their bonding. Compare the elect
Designing an Experiment: Investigating Bonding in Alloys
This question requires you to design an experiment that studies how alloy composition affects electr
Determining Molecular Polarity from Lewis Structures
Using the molecular geometry and Lewis structure of carbon dioxide ($$CO_2$$), determine its polarit
Evaluating Bond Energies and Reaction Enthalpy in Water Formation
This question requires the use of bond enthalpies to calculate the overall reaction enthalpy for wat
Evaluating Resonance Structures Using Formal Charge
Analyze the resonance structures of sulfur trioxide ($$SO_3$$) by calculating formal charges and dis
Expanded Octets and Molecular Stability in $$SF_6$$
A recent publication asserts that molecules like $$SF_6$$, which exhibit expanded octets, are except
Formal Charge Misinterpretation via Titration Experiment
A student attempts to determine the formal charge distribution in a polyatomic ion by performing an
FRQ 10: Bonding and Electrical Conductivity Across Phases
The following table summarizes the electrical conductivity of substances with different types of bon
FRQ 13: Polar Covalent Bonding – Electronegativity and Bond Strength
A table provides bond lengths and bond energies for several diatomic molecules with either nonpolar
FRQ 15: Determining Bond Order from Resonance Structures
A molecule with multiple resonance structures is described in the stimulus. In your answer, (a) desc
FRQ 17: Evaluating Bonding Effects on Solubility
A table lists the solubility data for various ionic and molecular covalent substances in water. In y
Graphite Melting Point Experiment with Network Covalent Solids
A student attempts to determine the melting point of graphite by placing a graphite sample in a high
Ionic Lattice Energy and Coulomb's Law
A solid ionic compound’s lattice energy can be estimated using Coulomb’s law. For an ionic compound,
Ionic vs Molecular Conductivity in Different Phases
This question asks you to analyze how different types of bonding affect electrical conductivity in v
Lewis Structure and Formal Charge of Carbonate Ion
A research paper discusses the carbonate ion $$CO_3^{2-}$$ and its resonance structures. Your task i
Measuring Bond Strength via Differential Scanning Calorimetry (DSC)
A research group employs differential scanning calorimetry (DSC) to determine the bond dissociation
Molecular Covalent Bonding and Lewis Structures
This question explores covalent bonding by requiring the construction of Lewis structures and the ca
Advanced Chromatographic Analysis
Analyze column chromatography data to compute separation factors and suggest modifications to improv
Analysis of Contact Angle Measurements and Intermolecular Forces
A student performs an experiment to study how intermolecular forces influence the behavior of liquid
Analysis of Ideal Gas Law Behavior under Varying Conditions
Examine the experimental conditions provided in the data table and analyze how pressure, volume, and
Chromatography and Separation of Mixtures
Consider a laboratory experiment utilizing paper chromatography to separate a mixture of colored dye
Combined Gas Law Application
A sample of gas initially has a pressure $$P_{1}= 2\ \text{atm}$$, a volume $$V_{1}= 10\ \text{L}$$,
Dalton's Law of Partial Pressures
Examine Dalton's law and its application to gas mixtures.
Determining Molecular Polarity and Dipole Moments
This question addresses the concepts of molecular geometry, bond polarity, and vector addition to de
Effusion and Gas Law Calculations
Investigate the relationship between molecular mass and the rate of effusion of gases, and apply gas
Effusion Experiment Analysis
Analyze the effusion behavior of gases to understand how molar mass influences the rate of effusion
Explaining Deviations from Ideal Gas Behavior
Real gases show deviations from ideal behavior under certain conditions. Provide both qualitative an
Gas Density and Molar Mass Determination
A researcher uses measurements of a gas's density to determine its molar mass through the ideal gas
Hydrogen Bonding and Its Effect on Physical Properties
This question focuses on the role of hydrogen bonding in determining physical properties. Use your u
Ideal Gas Law and Combined Gas Law Applications
Apply the ideal gas law and the combined gas law to analyze changes in gas systems.
Ideal Gas Law and Stoichiometry
A 10.0 L container is said to hold a mixture of three gases at 1.00 atm and 298 K: 0.50 mol of hydro
Impact of Hydrogen Bonding on Physical Properties
Hydrogen bonding can dramatically influence physical properties such as boiling point, melting point
Integrative Analysis of a Compound's Properties
A research team is tasked with an in-depth analysis of a newly synthesized compound. Their goal is t
Intermolecular Forces and Separation Techniques
A chemical engineer is tasked with separating a mixture of two liquids that differ in their predomin
Intermolecular Forces and Vapor Pressure
The strength of intermolecular forces (IMFs) has a direct impact on the vapor pressure of a liquid.
Intermolecular Forces and Vapor Pressure: A Multi-Phase Analysis
A researcher is examining how differences in intermolecular forces affect the vapor pressure of liqu
Investigation of Gas Effusion Rates
A student designs an experiment to compare the effusion rates of two gases by using a container with
Investigation of Vapor Pressure Variation
A student is investigating the effect of temperature on the vapor pressure of a volatile liquid. In
Kinetic Analysis of a Reaction via Spectrophotometry
A student studies the kinetics of a unimolecular reaction by monitoring the decrease in reactant con
Kinetic Molecular Theory and Root-Mean-Square Speed
Argon gas (molar mass = 39.95 g/mol) at 298 K is contained in a vessel. Answer the following:
Maxwell-Boltzmann and Kinetic Energy Analysis
Utilize the Maxwell-Boltzmann graph to analyze the effects of temperature and molecular mass on the
Maxwell-Boltzmann Distribution Analysis
Examine the Maxwell-Boltzmann graph provided, which shows molecular speed distributions for a gas at
Molecular Dipole Analysis
Molecular polarity is critical to the behavior of substances. Two molecules – Molecule A (tetrahedra
Molecular Polarity Analysis
A chemist is investigating how molecular geometry influences molecular polarity using the molecules
Molecular Polarity and Dipole Interactions
Examine the molecular structures of NF3 and CF4. Using your knowledge of polar covalent bonds and mo
Paper Chromatography and Retention Factor Analysis
Paper chromatography separates a mixture based on differences in polarity. Analyze the separation us
Paper Chromatography Pigment Separation Analysis
Examine the experimental results from a paper chromatography experiment used to separate pigments. U
Pressure-Temperature-Volume Relationships
In a controlled experiment, a gas is subjected to varying pressures and temperatures. Answer the fol
Solvent Polarity and Solubility Relationship
The principle 'like dissolves like' suggests that polar solvents are more effective at dissolving po
Acid-Base Reaction Efficiency Analysis
A series of experiments were conducted to study the efficiency of acid-base neutralization reactions
Ammonia Synthesis Reaction Analysis
The Haber process for ammonia synthesis is represented by: $$N_2(g) + 3H_2(g) \leftrightarrow 2NH_3(
Assessing Percent Error in Stoichiometric Calculations
Design an experiment to compare the theoretical and experimental yields in a synthesis reaction and
Combustion Analysis of a Hydrocarbon
A 3.00 g sample of an unknown hydrocarbon is combusted in excess oxygen, producing 12.0 g of carbon
Combustion Analysis: Determining Empirical Formula of a Hydrocarbon
Design an experiment to perform combustion analysis of an unknown hydrocarbon. Your procedure should
Comparing Reaction Yields: Theoretical vs. Actual
In a reaction, the theoretical yield of a product is calculated to be $$12.0$$ g, but the experiment
Decomposition Reaction Analysis
A compound $$AB$$ is known to decompose upon heating, following the reaction: $$AB \rightarrow A + B
Determining the Limiting Reactant in a Redox Reaction
In redox reactions, the limiting reactant dictates the reaction’s extent. Design an experiment using
Electrochemistry: Balancing Redox Reactions in Acidic Medium
In acidic solution, permanganate ions oxidize iron(II) ions according to the reaction: $$MnO_4^{-} +
Experimental Design: Reaction Rate and Stoichiometry
Design an experiment to investigate the reaction rate of a synthesis reaction where multiple variabl
FRQ 3: Gravimetric Analysis of an Unknown Alkali Hydroxide
A 4.33 g sample of an unknown alkali hydroxide is completely dissolved and reacted with copper(II) n
Gravimetric Analysis for Alkali Hydroxide Identification
An unknown alkali hydroxide compound sample weighing 4.33 g is dissolved in water and reacted with a
Gravimetric Analysis for Determining Unknown Alkali Hydroxide
A 4.33 g sample of an unknown alkali hydroxide is dissolved in water and reacted with $$Cu(NO_3)_2$$
Gravimetric Analysis for Unknown Composition
A gravimetric analysis experiment is performed to determine the percentage of chloride in a sample b
Gravimetric Analysis of an Unknown Salt
An unknown salt that contains chloride ions is analyzed by precipitating AgCl from a 1.00 g sample u
Investigation of Synthesis Reaction Yield
An experiment was conducted to study a synthesis reaction represented by A + B → AB under varying te
Percent Error Analysis in Experimental Chemistry
In an experiment, the theoretical yield of a product was calculated to be $$2.50$$ g, while the actu
Percent Error Analysis in Reaction Yield
In three separate reaction trials, the experimental yield and theoretical yield were recorded. Revie
Precipitation Reaction and Solubility Rules
A 50.0 mL solution of 0.50 M AgNO3 is mixed with 50.0 mL of 0.50 M NaCl in a 100.0 mL container. A p
Precipitation Reaction: Formation of Silver Chloride
Silver nitrate reacts with sodium chloride in aqueous solution to form silver chloride precipitate a
Quantitative Analysis With Percent Error
Percent error is used to compare experimental yields with theoretical predictions. Design an experim
Reaction Kinetics of a Decomposition Reaction
The decomposition of compound A follows first order kinetics. The following table provides the conce
Reaction Kinetics: Half-Life and First-Order Reactions
A radioactive substance decays following first-order kinetics with a half-life of 8.00 hours.
Redox Reaction and Oxidation States
Consider the redox reaction between copper(II) ions and metallic iron: $$Cu^{2+}(aq) + Fe(s) \righta
Redox Titration Data for Determining Concentration of an Unknown
A redox titration is performed using a 0.05 M KMnO₄ solution to determine the concentration of an un
Solubility and Precipitation Reactions Analysis
Consider the precipitation reaction: $$A (aq) + B (aq) \rightarrow C (aq) + D (s)$$, and the solubil
Advanced Kinetic Analysis with Competing Reaction Pathways
Consider a reaction in which the reactant A can follow two parallel pathways with rate constants $$k
Analysis of a Reaction Energy Profile Diagram
An energy profile diagram of a chemical reaction is provided, which shows the energy levels of react
Analysis of Reaction Kinetics Using Initial Concentration Data
A reaction, $$A + 2*B + C \to D$$, was investigated using a series of experiments. The following tab
Analyzing Rate Law from Concentration Doubling Experiments
A reaction follows the rate law $$\text{Rate} = k [X]^m [Y]^n$$. The following experimental data wer
Analyzing the Impact of Reaction Thermodynamics on Kinetics: Endothermic vs. Exothermic Steps
A reaction mechanism involves an initial endothermic step followed by an exothermic step. An energy
Catalysts in Reaction Mechanisms
Consider the reaction A + B → C that occurs via the following mechanism: I. A + X → Y II. B + Y → C
Comparative Graph Analysis for Integrated Rate Laws
An experiment was conducted to determine the order of a reaction by plotting different transformatio
Complex Effects of Reactant Concentration on Reaction Mechanisms
A reaction is studied by varying the concentrations of reactants A and B. The following data were ob
Derivation of Integrated Rate Laws
Derive the integrated rate laws for first-order and second-order reactions starting from their respe
Designing an Experiment to Investigate the Effect of Temperature on Reaction Rates
A chemist is designing an experiment to study how temperature affects the reaction rate of the react
Determination of Activation Energy Using the Arrhenius Equation
A chemist conducted experiments to study the temperature dependence of a reaction. The following dat
Determining Half-Life for a First-Order Reaction
A first-order reaction is monitored and the concentration of reactant A is measured over time. The e
Effects of Reactant Concentration Changes on Reaction Rates
A series of experiments was performed on the reaction $$F + G \to H$$ where the concentration of rea
Elucidating Reaction Mechanism and Rate-Determining Step
Consider the following elementary reaction mechanism: I. $$A + A \rightarrow X$$ (fast) II. $$X +
Exploring Reaction Temperature Effects Using Arrhenius Plots
A series of experiments were conducted to measure the rate constant $$k$$ of a reaction at different
Exploring the Role of Molecular Orientation in Collision Theory
A team of researchers is investigating why not all molecular collisions lead to a reaction. They foc
Half-Life and Reaction Order Determination
Half-life is a useful concept in reaction kinetics. Answer the following parts to explore its implic
Integrated Rate Law Analysis for a Second Order Reaction
A second order reaction was investigated by monitoring the concentration of reactant A over time. Th
Interpreting Reaction Energy Profiles
A reaction energy profile diagram is provided below. The diagram shows the energy of the reactants a
Investigating the Effect of Pressure on Gas-Phase Reaction Rates
A chemist investigates the decomposition of a gaseous compound by varying its pressure while keeping
Investigation of Rate Law using Initial Concentration Measurements: Error Analysis
A research team investigated the reaction $$A + 2*B + C \rightarrow D$$ by measuring the initial rat
Misidentification of the Rate-Determining Step in Multi-Step Reactions
An experiment was conducted on a multi-step reaction where the mechanism involves both fast and slow
Photochemical Reaction Kinetics in a Light-Activated Reaction
Certain reactions are initiated by the absorption of light (photochemical reactions).
Synthesis Investigation: Determining Reaction Orders in a Complex Reaction
Investigate the kinetics of the complex reaction $$2X + Y \rightarrow Z$$. Using initial rate data g
Temperature Measurement Calibration in Arrhenius Plot Construction
An experiment aimed at determining the activation energy of a reaction measured rate constants at di
The Effect of Temperature on Reaction Kinetics
A chemical reaction was studied at several temperatures. The following table lists the measured rate
Zero-Order Reaction Kinetics Analysis
A reaction is suspected to follow zero-order kinetics. The concentration of reactant A was measured
Analysis of Reaction Energy Diagram with Intermediates
An energy diagram is provided for the multi-step reaction $$A → B → C$$. The diagram indicates that
Analyzing Enthalpy of Solution
When an ionic salt dissolves in water, the process involves several energetic changes. The dissoluti
Bomb Calorimeter for Combustion Reactions
A student uses a bomb calorimeter to determine the enthalpy of combustion of a fuel sample. In the e
Calorimetry in Action
A reaction in a coffee-cup calorimeter results in a temperature change in the surrounding water. Sup
Calorimetry of an Aqueous Reaction
An aqueous reaction was carried out using a coffee-cup calorimeter. A 120.0 g solution experienced a
Calorimetry: Determining Specific Heat
Calorimetry is used to determine the specific heat capacity of an unknown metal in an experimental s
Catalyst Efficacy in Lowering Activation Energy
A student investigates how varying the concentration of a catalyst affects the reaction time of a ch
Catalyst Impact on Activation Energy and Reaction Kinetics
A researcher studies a reaction both in the presence and absence of a catalyst. The reaction kinetic
Catalyst Recycling and Energy Savings in Industrial Processes
An industrial chemical process employs a catalyst to reduce the energy consumption of a reaction. Th
Comparing Enthalpy of Fusion and Vaporization
Discuss the energetic requirements for phase transitions from solid to liquid (fusion) and liquid to
Comparing Theoretical and Experimental Enthalpy Changes
A reaction was studied using both theoretical bond energy calculations and experimental calorimetry.
Designing a Calorimetry Experiment to Measure Enthalpy of Fusion
Develop an experimental procedure to accurately measure the enthalpy of fusion for an unknown substa
Determining Reaction Enthalpy from Bond Energies
A claim is made that the enthalpy change of a reaction can be accurately determined by using average
Determining Reaction Enthalpy using Bond Energies
For the synthesis reaction of ammonia: $$\text{N}_2 + 3\,\text{H}_2 \rightarrow 2\,\text{NH}_3$$ cal
Determining Specific Heat Capacity of a Metal
A student aims to measure the specific heat capacity of an unknown metal. The procedure involves hea
Effect of a Catalyst on Reaction Energy Diagram
An energy diagram is provided for a chemical reaction conducted both with and without a catalyst. Th
Enthalpy of Solution: Ammonium Nitrate Dissolution
A student attempts to determine the enthalpy change for dissolving ammonium nitrate (NH4NO3) in wate
Enthalpy of Solution: Dissolution of an Ionic Compound
In a calorimetry experiment, 5.00 g of ammonium nitrate is dissolved in 100.0 g of water inside a ca
Evaluating Experimental Design in Calorimetry
A research group designed an experiment to measure the enthalpy change of a neutralization reaction
Experimental Design: Measuring Activation Energy
A media report claims that temperature measurements from a reaction vessel can directly determine th
Formation of Methanol and Enthalpy of Formation Analysis
Consider the reaction for the formation of methanol: $$C(s) + \frac{1}{2} O_2(g) + 2 H_2(g) \rightar
Heat and Temperature: Conceptual Distinctions
Heat and temperature are often confused despite being distinct concepts. Using your understanding of
Interpreting a Temperature vs. Time Graph During a Phase Change
A graph of temperature versus time during a phase change is provided. Analyze the graph to explain t
Modeling Energy Diagrams for Endothermic Reactions
In a study of an endothermic reaction, researchers use temperature measurements to construct an ener
Phase Change Calorimetry Experiment Design
Design an experiment using calorimetry to measure the enthalpy of fusion of ice.
Thermodynamics of Sublimation
Dry ice (solid CO$_2$) sublimates directly into a gas at atmospheric pressure. A researcher investig
Acid-Base Equilibria: Calculating Ka and pKa
Consider the weak acid $$HA$$ which dissociates as $$HA \leftrightarrow H^{+} + A^{-}$$.
Application of Le Chatelier's Principle in Industrial Settings
The Haber process for ammonia production is represented as: $$N_2(g) + 3\,H_2(g) \rightleftharpoons
Base Dissociation Constant (Kb) of Ammonia: Error in Water Concentration Assumption
In an experiment to determine the base dissociation constant (Kb) for ammonia, the reaction $$NH_3 +
Concentration Effects in the Haber Process
The Haber synthesis reaction for ammonia is given by $$N_2(g)+3H_2(g) \rightleftharpoons 2NH_3(g)$$.
Determining Acid Dissociation Constant (Ka)
For the weak acid HA, which dissociates in water according to $$HA + H_2O \leftrightarrow A^- + H_3O
Determining the Reaction Quotient in an Aqueous Equilibrium Reaction
For the equilibrium reaction: $$M(aq) + N(aq) \rightleftharpoons P(aq)$$, the system is not initiall
Dilution Effects on Aqueous Equilibrium
For the equilibrium reaction $$\text{Fe}^{3+} (aq) + \text{SCN}^- (aq) \leftrightharpoons \text{FeSC
Direct Measurement of Reaction Rates Near Equilibrium
Design an experiment to directly measure the forward and reverse reaction rates as the system approa
Dynamic Analysis of Reaction Quotient Over Time
A system undergoing the reaction $$\mathrm{D (aq) + E (aq) \rightleftharpoons F (aq)}$$ is monitored
Equilibrium Behavior Under Non-Ideal Conditions
In real solutions, non-ideal behavior such as ionic strength can affect the measured equilibrium con
Equilibrium Data Analysis in a Laboratory Experiment
In a lab experiment, the following data were collected for the reaction $$Fe^{3+}(aq) + SCN^-(aq) \l
Equilibrium Expression and Concentration Effects
For a general reaction $$aA + bB \leftrightarrow cC$$, answer the following:
Exploring Equilibrium Expressions
For the general reaction $$a*A + b*B \leftrightarrow c*C + d*D$$, answer the following parts.
Gas-Liquid Equilibrium: Henry’s Law and Reaction Equilibrium
Consider the reaction: $$O_2(aq) + 2X(aq) \rightleftharpoons Y(aq)$$, where the solubility of O₂ in
Graph Analysis of Equilibrium Attainment
A graph depicting the concentration of reactants and products over time for a reversible reaction is
Graphical Analysis of Reaction Equilibria
A study investigates the simple reaction $$A \rightleftharpoons B$$. The concentration of A decrease
Haber Process Equilibrium Analysis
The Haber process converts N₂ and H₂ into NH₃ in a reversible reaction. Analyze the provided graph f
Haber Process Equilibrium Calculation
In the Haber process, $$N_2 + 3*H_2 \leftrightarrow 2*NH_3$$, a container initially contains $$[N_2]
Hess's Law and Composite Equilibria
Consider the following reactions with their respective equilibrium constants: (1) $$A (g) \rightlef
Hess's Law and Equilibrium Constant Combination: Error Identification
A researcher seeks to determine the overall equilibrium constant for a composite reaction by combini
Hess's Law and Equilibrium Constant Manipulation
A chemist is analyzing how equilibrium constants change when individual reactions are manipulated. A
Impact of Common Ion on Solubility Equilibrium
A chemist is studying the solubility of silver chloride (AgCl) and the impact of a common ion on its
Impact of Dilution on Aqueous Equilibrium: The FeSCN2+ System
The equilibrium reaction for the formation of the colored complex ion is given by: $$Fe^{3+}(aq) + S
Kp and Kc Interconversion
For the reaction: $$N_2 (g) + 3*H_2 (g) \rightleftharpoons 2*NH_3 (g)$$, answer the following parts:
Manipulating Equilibrium Constants: Reaction Reversal and Multiplication
Given the reaction $$\text{A} + 2*\text{B} \leftrightharpoons 3*\text{C}$$ with an equilibrium const
Manipulating Equilibrium Expressions Using Hess's Law
The following reactions are at equilibrium: (1) $$A \rightleftharpoons B$$ with $$K₁ = 4.0$$ (2) $$B
Manipulation of Equilibrium Constant via Hess's Law
Consider the following reactions: Reaction (1): $$A \rightleftharpoons B$$ with $$K_1 = 2.0$$ Reac
Manipulations of Equilibrium Constants using Hess's Law
Examine how equilibrium constants transform when the reaction is manipulated. Answer the following p
Oxygen Binding in Hemoglobin: An Equilibrium Perspective
Hemoglobin binds oxygen reversibly, and its binding equilibrium can be influenced by changes in carb
Pressure and Volume Effects on Equilibrium
Consider the following equilibrium reaction: $$2NO_2 (g) \rightleftharpoons N_2O_4 (g)$$. Answer the
Reaction Mechanism and Intermediate Analysis
For the reaction mechanism involving two steps: Step 1: $$A (g) \rightleftharpoons B (g)$$ (fast equ
Reaction Quotient Analysis
In an experiment on the reaction $$A + 2*B \rightleftharpoons 3*C$$, initial concentrations are meas
Reaction Quotient Calculations for NO and NO2
For the equilibrium reaction: $$2NO (g) + O_2 (g) \rightleftharpoons 2NO_2 (g)$$, consider the initi
Relating Concentration and Pressure Equilibrium Constants
Differentiate between the concentration-based equilibrium constant $$K_c$$ and the pressure-based eq
Solubility and Precipitation Reactions
Consider the dissolution equilibrium of barium sulfate: $$BaSO_4 (s) \rightleftharpoons Ba^{2+} (aq)
Solubility and the Common Ion Effect
The solubility equilibrium for silver chloride is given by: $$AgCl(s) \rightleftharpoons Ag^+(aq) +
Solubility Product and Precipitation Prediction
For the dissolution reaction of lead(II) chloride: $$\mathrm{PbCl_{2} (s) \rightleftharpoons Pb^{2+}
Solubility Product and the Common Ion Effect
Consider the dissolution of the salt $$AgCl(s) \leftrightarrow Ag^{+}(aq) + Cl^{-}(aq)$$.
Temperature Effects in an Exothermic Equilibrium
For the exothermic reaction Y ⇌ Z + energy, experiments were conducted at various temperatures. The
Acid-Base Titration Curve Analysis for HCl and NH₃
An experiment involves titrating a strong acid (HCl) with a weak base (NH₃). A graph of pH versus vo
Analysis of Polyprotic Acid Titration
A titration experiment is conducted using phosphoric acid (H3PO4), a polyprotic acid, with a strong
Buffer Response in a Weak Acid–Strong Base Titration
During the titration of a weak acid with a strong base, the solution exhibits a buffer region where
Buffer System Experiment Design
A chemistry student aims to prepare a buffer solution using acetic acid (HC2H3O2) and its conjugate
Common Ion Effect and Its Impact on Solubility
The solubility of sparingly soluble salts can be substantially affected by the common ion effect.
Common Ion Effect Misinterpretation in Solubility
An experiment was conducted to determine the solubility of Mg(OH)₂ by dissolving it in solutions of
Common Ion Effect on Solubility of Mg(OH)₂
This question examines the impact of the common ion effect on the solubility of magnesium hydroxide.
Computational Modeling of Acid-Base Equilibria
A researcher develops a computer simulation to model complex acid-base equilibria in aqueous solutio
Determining pKb from a Known pKa
A study of conjugate acid-base pairs involves using the relation $$pK_a + pK_b = 14$$. For acetic ac
Dilution Effects on Weak Acid Equilibrium
Explain how diluting a solution of a weak acid affects its percent dissociation and pH. Support your
Indicator and Titration Endpoint Determination
Indicators are used to detect the endpoint in titrations. Answer the following:
Interpreting pH Variability in Environmental Waters
A researcher collects pH data from several lakes, finding values ranging from 6.2 to 8.4. Discuss th
Mixing Strong and Weak Acid Solutions
A chemist mixes 50.0 mL of 0.10 M HCl (a strong acid) with 50.0 mL of 0.10 M acetic acid (a weak aci
Neutralization Reaction Error in Weak Base Titration
A student performed a titration of ammonia (NH₃), a weak base, with a strong acid (HCl). The student
Percent Dissociation of a Weak Acid
Consider a 0.10 M solution of acetic acid (HC₂H₃O₂) with a dissociation constant $$K_a = 1.8 \times
pKa, pKb, and Their Relationship in Acid-Base Equilibria
The relationship between $$pK_a$$ and $$pK_b$$ for a conjugate acid-base pair is a fundamental conce
Polyprotic Acid Titration and Stepwise Dissociation
Phosphoric acid (H3PO4) is a polyprotic acid that dissociates in three steps with the dissociation c
Predicting pH Changes When Mixing a Strong Acid with a Buffer
This FRQ examines how the addition of a strong acid to a buffer solution affects the pH, using exper
Quantitative pH Analysis with Percent Dissociation: Calculation Error
A student investigated the percent dissociation of acetic acid in a dilute aqueous solution by measu
Using an ICE Table for pH Calculation of a Weak Acid
Acetic acid, a weak acid, partially dissociates in water. Consider a 0.1 M acetic acid solution with
Water Ionization and pH Extremes at Elevated Temperatures
A study examines how temperature affects the ionization of water. It is claimed that at temperatures
Water Ionization and Temperature Effects
This question explores the ionization of water and the effect of temperature changes on the ion prod
Weak Acid Equilibrium ICE Analysis
A 0.10 M solution of the weak acid HA has an acid dissociation constant of $$K_a = 1.0 \times 10^{-5
Weak Acid Percent Dissociation Analysis
Consider a 0.050 M acetic acid solution with a dissociation constant $$K_a = 1.8 \times 10^{-5}$$. T
Calculating Moles Electrolyzed in a Process
An electrolytic cell is operated at a constant current to deposit silver. The cell runs at 5.00 A fo
Catalysts and Reaction Free Energy
Catalysts increase the rate of a reaction without altering its thermodynamic properties. Analyze thi
Combining Half-Reactions and Calculating E°
Using the following half-reactions: (1) $$\text{MnO}_4^- + 8H^+ + 5e^- \rightarrow \text{Mn}^{2+} +
Comparing Spontaneity in Redox vs. Non-redox Reactions
A complex chemical process involves coupling a spontaneous redox reaction with a non-spontaneous rea
Corrosion Protection via Sacrificial Anodes
A coastal facility experiences rapid corrosion of aluminum structures. An engineer proposes using a
Designing an Experiment to Measure Entropy Change
Design a laboratory experiment that aims to measure the entropy change when mixing two aqueous solut
Effect of Non-Standard Conditions on Cell Voltage Using the Nernst Equation
Consider the reaction $$Zn(s) + Cu^{2+}(aq) \rightarrow Zn^{2+}(aq) + Cu(s)$$. Using the Nernst equa
Effect of Temperature on Gibbs Free Energy: Data Analysis
An experiment records the variation in Gibbs free energy (\(\Delta G\)) of a reaction with temperatu
Electrochemical Cell Potential from Reduction Potentials
Consider the redox couple given by the following half-reactions: 1. $$\text{Cu}^{2+} + 2e^- \rightar
Enhancing Efficiency in Electrolytic Processes
An industrial electroplating process is being optimized to increase efficiency and achieve uniform m
Entropy and Reaction Disorder Analysis
This question examines the concept of entropy change and reaction disorder in chemical reactions.
Entropy and Reaction Spontaneity Analysis
A recent media report claims that reactions in which the number of gas moles increases (for example,
Entropy Calculation from Reaction Data
A researcher is investigating the change in entropy (S) for a reaction occurring under standard cond
Error in Linking Electrochemical Potentials to Gibbs Free Energy
In an experiment aimed at correlating measured electrochemical cell potentials with Gibbs free energ
FRQ 2: Temperature Dependence of Gibbs Free Energy
Consider a reaction for which the standard enthalpy change is \(\Delta H^\circ = -100\,kJ/mol\) and
Gibbs Free Energy Correction Under Non-Standard Conditions
A redox reaction is observed under non-standard conditions and its cell potential is lower than the
Gibbs Free Energy under Non-Standard Conditions
An experiment examines how deviations from standard conditions affect the cell potential in an elect
Graphical Analysis of Reaction Energy Profiles
An energy profile diagram for a chemical reaction is provided. The diagram shows the energy levels o
Investigating Temperature Dependence of Gibbs Free Energy
An experiment provides data on the free energy change of a reaction at different temperatures. A tab
Kinetic vs Thermodynamic Control in Reactions
An experimental study investigates a reaction that can be under either kinetic control or thermodyna
Kinetics vs Thermodynamics in Electrochemical Cells
A study claims that the rate of an electrolysis process is determined solely by its thermodynamic fa
Non-Standard Conditions and the Nernst Equation
Consider a redox reaction operating under non-standard conditions. Analyze how deviations from stand
Predicting Changes in Voltage Due to Variations in Conditions
A galvanic cell has an initial standard cell potential of $$E^\circ_{cell} = 1.25\,V$$ under 1 M con
Predicting Spontaneity in Redox Reactions
Given the following half-reactions and their standard reduction potentials: (a) $$\text{Fe}^{3+} + e
Pressure Effects on Gibbs Free Energy
In gas-phase reactions, pressure changes can influence the Gibbs free energy. Consider a reaction th
Redox Reaction Analysis in a Galvanic Cell
A galvanic cell is constructed with copper and zinc electrodes. The standard reduction potentials ar
Redox Reaction Analysis in Acidic Media
In an electrochemical cell operating in an acidic medium, the following half-reactions are provided:
Thermodynamic Analysis of a Complex Reaction
A complex reaction is studied under varying conditions to understand the interplay between enthalpy,
Trusted by millions
Everyone is relying on Knowt, and we never let them down.



Need to review before working on AP Chemistry FRQs?
We have over 5 million resources across various exams, and subjects to refer to at any point.
Browse top AP materials
We’ve found the best flashcards & notes on Knowt.
Explore top AP notes
Tips from Former AP Students
FAQ
We thought you might have some questions...
- Of course, make sure to run through College Board's past FRQ questions!
- Once you’re done with those go through all the questions in the AP ChemistryFree Response Room. You can answer the question and have it grade you against the rubric so you know exactly where to improve.
- Reddit it also a great place to find AP free response questions that other students may have access to.