d block chemistry
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
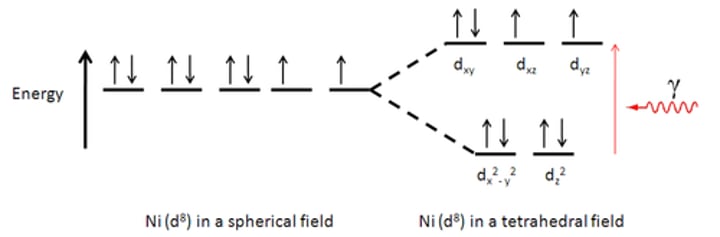
describe the splitting of a tetrahedral complex
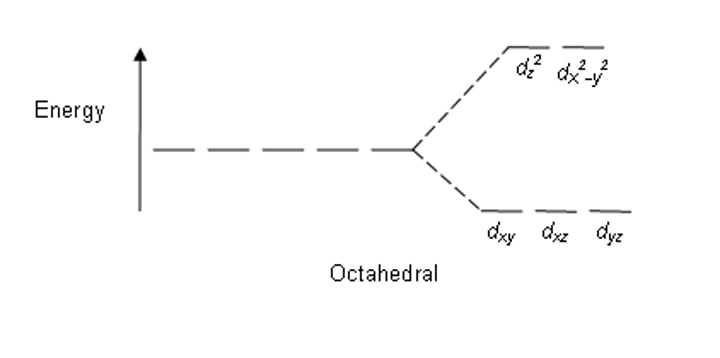
describe the splitting of an octahedral complex
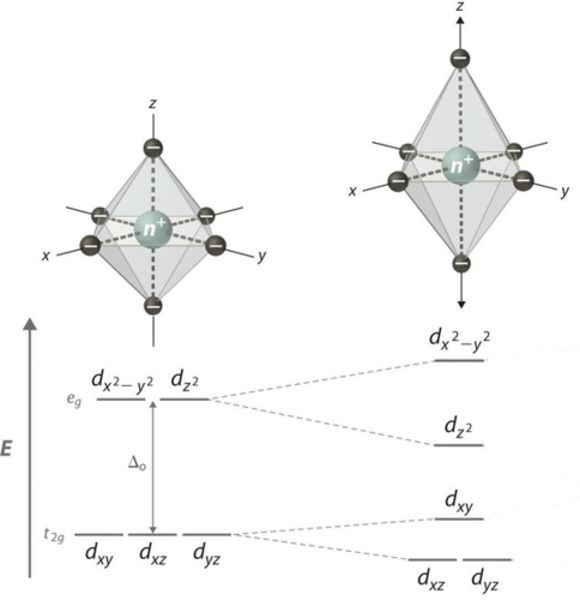
describe the Jahn Teller distortion - elongation
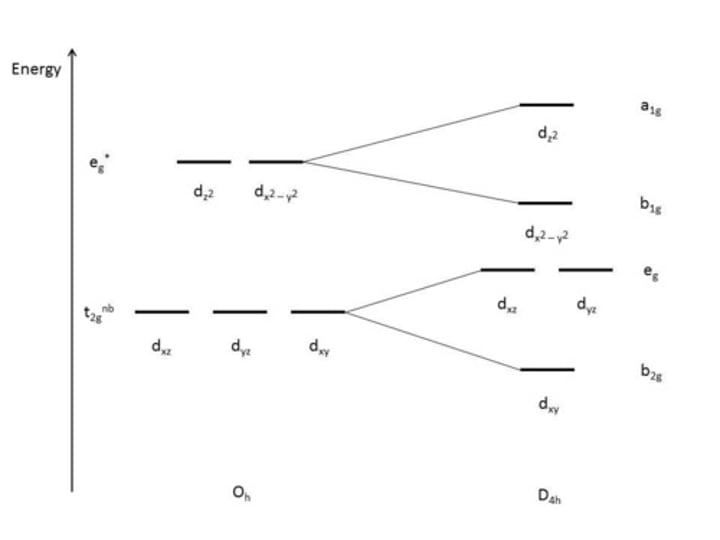
describe the Jahn Teller distortion - compression
describe the Jahn Teller effect
occurs when valence electron has choice of degenerate orbitals, further distortion of d orbitals from tetrahedral/octahedral arrangement creates non-degenerate orbitals, either compression or elongation: dz2 and dx2-y2 both become higher in energy
describe the splitting of a square planar complex
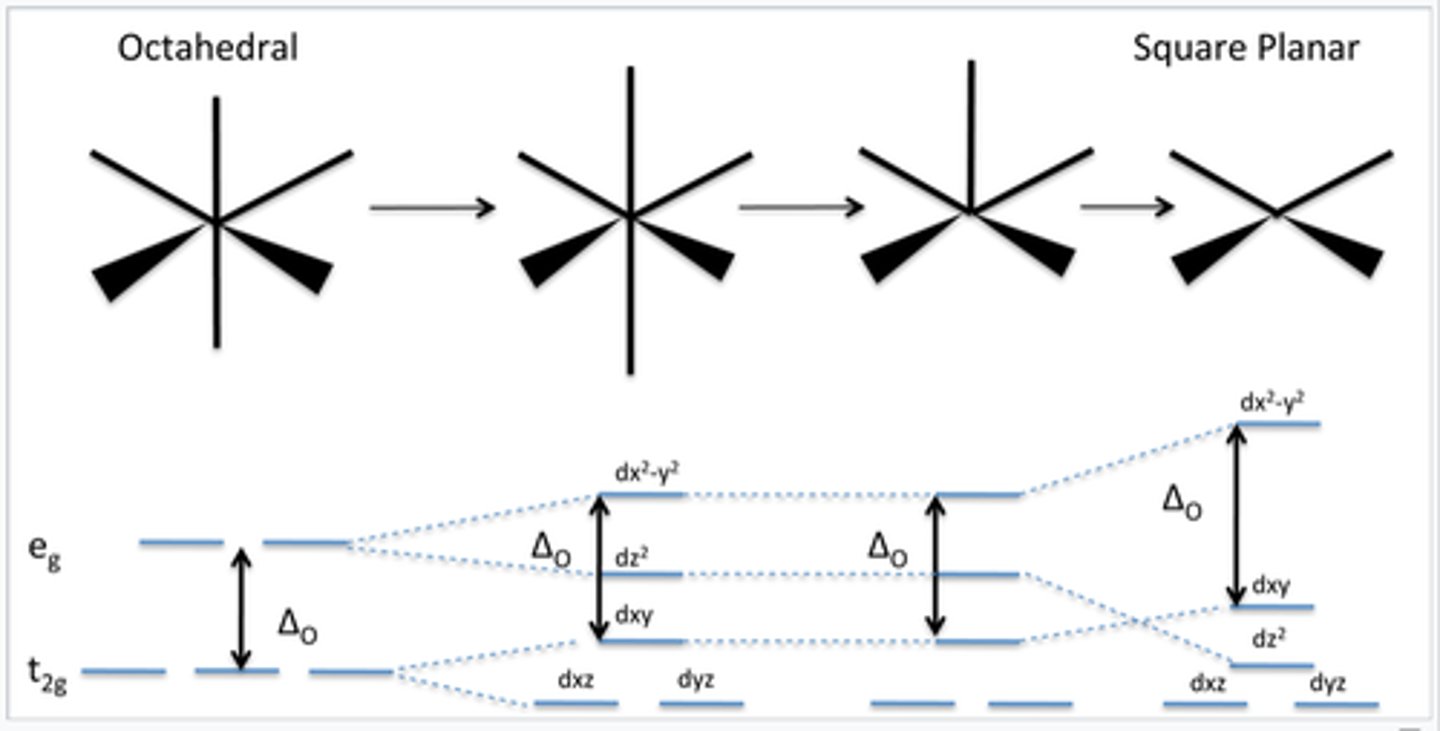
name some strong field ligands, (and their charges) what does this mean for the arrangement of electrons in a complex, why?
CN-, NO+, CO, > NO2(2-) > py, NH3 > NCCH3 strong field ligands cause large splitting between t(2g) and e(g) making them low spin because the E difference between eg and tg is greater than the E requirement to pair an electron (e repulsion)
name some weak field ligands (and their charges), what does this mean for the arrangement of electrons in a complex, why?
I- < Br-< SCN- < Cl- < F-, OH- < OH2
weak field ligands cause small splitting between e(g) and t(2g) so the energy requirement to pair an electron is greater than the energy between t(2g) and e(g), they are usually high spin
list the selection rules
Spin-spin selection
Laporte/parity
describe the Laporte/parity selection rule
for molecules with centre of symmetry, electronic transitions can only be made between u and g. g to g and u to u is forbidden. d orbitals are all g orbitals (have symmetry) so effectively d to d orbital transitions are not allowed.
this rule is not super strict as donation from lone pairs of electrons are allowed (charge transfer) e.g. in MnO4, oxygen reduces Mn and then donates electrons back (non-bonding e = lone pairs on O) so technically d to d transition but passed off as p to d transition
other exceptions: distorted octahedral complexes don't have as great symmetry, vibrations of bonds mean centre of symmetry can be lost temporarily, hybridisation of orbitals mean p to d transitions can occur
octahedral complexes are the only complexes with symmetry so this rule does not apply to tetrahedral/square planar complexes
describe the spin-spin selection rule
states electrons cannot change spin during a transition. e.g. when all orbitals singly occupied and electron promoted to higher orbital: changes spin
forbidden for all types of complexes
e.g. high spin d5
state the order of colour intensity dictate by rules (octahedral/tetrahedral/which rule etc.), which complex is usually more intensely coloured?
1. just spin-spin selection allowed (not d-d transitions) - octahedral
2. octahedral d-d transitions + spin
3. tetrahedral d-d transitions allowed + spin
tetrahedral complexes usually more intensely coloured
what are the exceptions to the usual d shell filling and why?
Cr, Mo = have 1 electron away from being half-filled, d orbitals are d orbitals which are less than one from half-filled/full are less stable than when half-filled/full
changes from [Ar] 4s2 3d4 to [Ar] 4s1 3d5 (half-full)
Cu, Ag = same principle applies except for full d shell
changes from [Ar] 4s2 3d9 to [Ar] 4s1 3d10
what is special about the SCN ligand? is it strong/weak, what's its charge?
charge = -1
strong field ligand, so produces low spin complexes
special because can coordinate from either S or N - produces linkage isomers
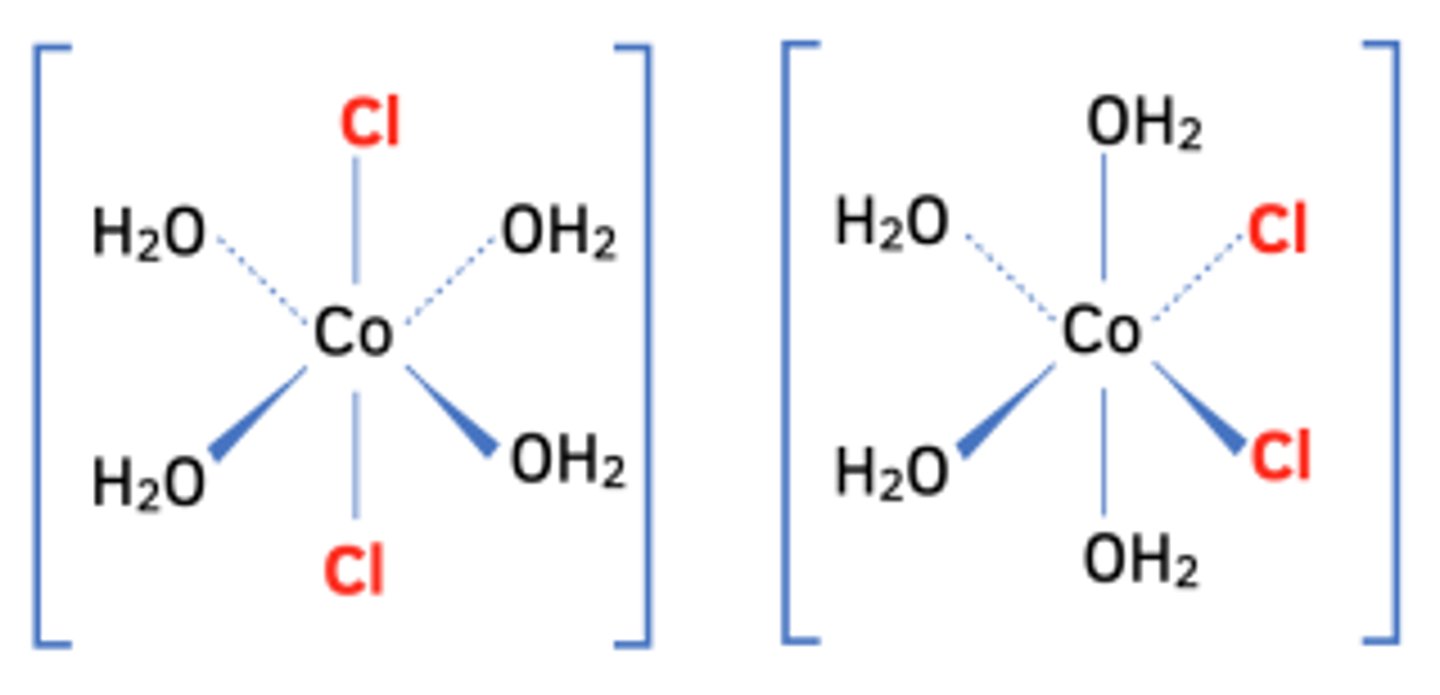
describe/explain trans/cis isomerism in TM complexes?
cis = when molecule is on the same side of central atom, only occurs with one molecule e.g. one Cl ligand, 3 NH3 ligands
trans = must cross central atom to get to other molecule
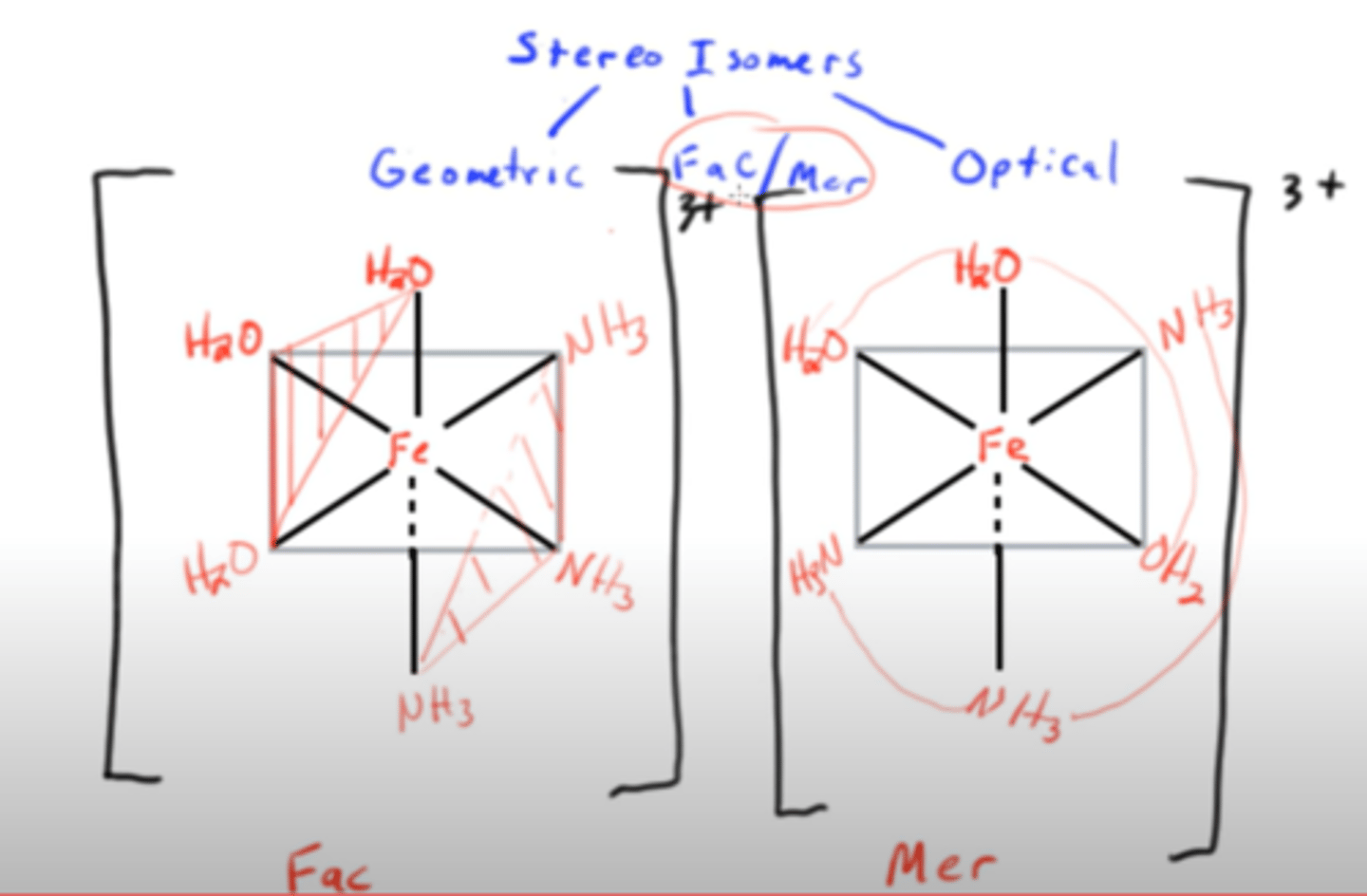
describe/explain fac/mer isomerism in TM complexes?
fac = in an octahedral complex 3 of the same ligands consecutively to make a triangle when connected
mer = broken up e.g. 2 NH3, then H2O, then NH3 to make a curved line
(see pic)
does the type of metal affect the spin of the complex?
yes - heavier (and bigger) metals with higher oxidation states tend to form only low spin complexes as their orbitals are more diffuse so overlap is stronger, making the energy gap bigger. bigger energy gap > electron repulsion energy so complexes are usually low spin.
only exception: PF2
how do you tell which colour a complex is?
the bigger the CFSE, the smaller the wavelength absorbed so the longer the frequency colour shown
bigger CSFE = octahedral and stronger field ligands (tetrahedral always smaller than octahedral and SF ligands always bigger than WF ligands)
E = hλ, c = vλ so E = ch/v - wavelength is small when E is big
c = vλ, so small λ = big frequency (v)
give the ranges of red wavelengths absorbed and what colour it will be
620 - 800 nm
appears blue
give the ranges of blue wavelengths absorbed and what colour it will be
430 - 490nm
appears red/pink/orange-y
give the ranges of green wavelengths absorbed and what colour it will be
490 - 560nm
appears violet - red (pink)
give the ranges of yellow wavelengths absorbed and what colour it will be
560 - 580nm
appears violet
give the ranges of violet wavelengths absorbed and what colour it will be
400 - 430nm
appears yellow
give the ranges of orange wavelengths absorbed and what colour it will be
580 - 620 nm
appears blue
what is the equation for ΔG involving the stability constant?
ΔG = -RTlnK
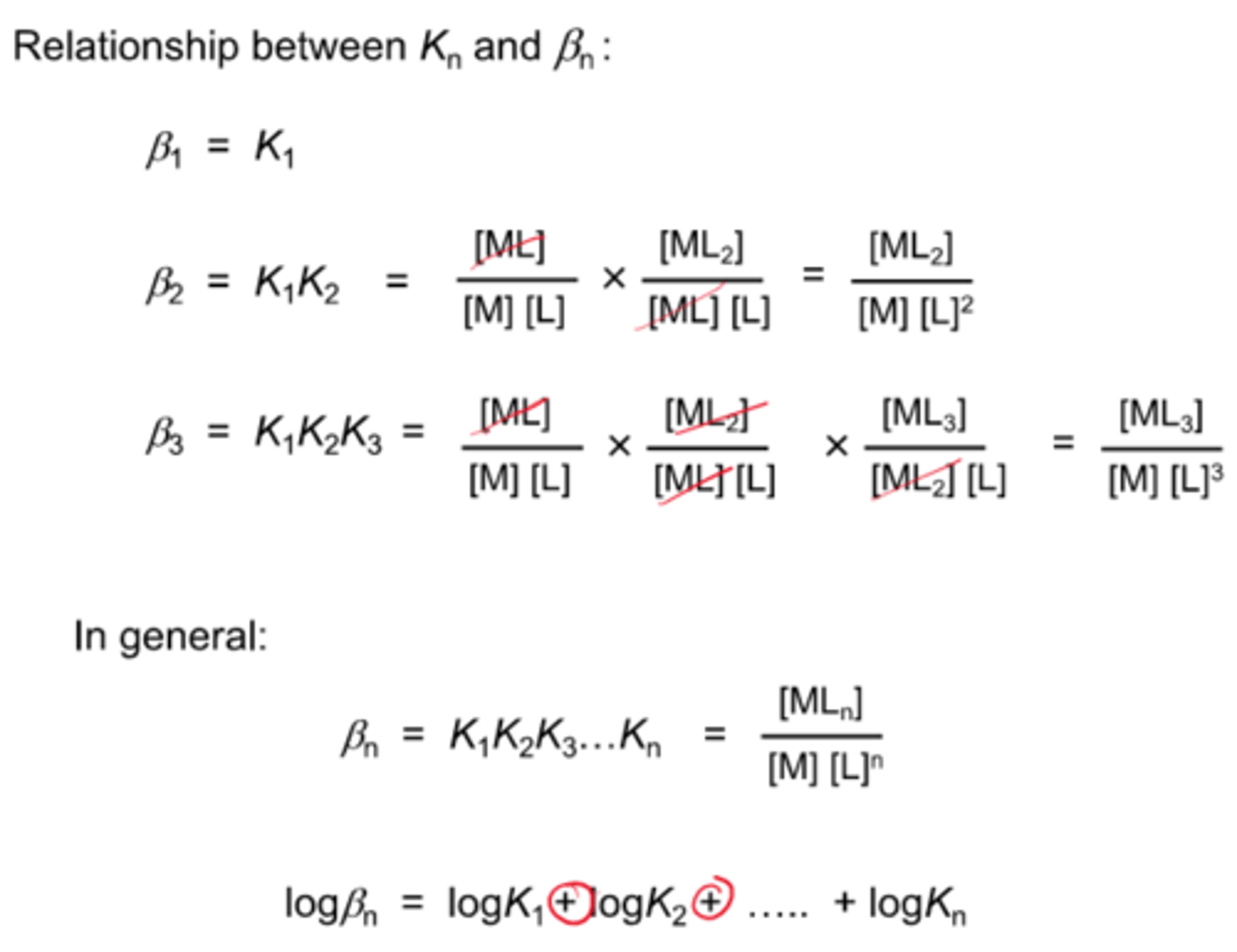
what is the equation for the overall stability constant?
β(initial) - β(final) (β is the same as K basically)
logβn = logK1 + logK2 + logK3 ... logKn
describe the chelate effect in terms of the stability constant, why does this happen?
the enhanced stability of a complex containing (polydentate) ligands over one containing similar monodentate ligands e.g. NH3 vs en (en 10 magnitudes larger)
most stable when 5/6 membered rings
increase in species on the right hand side = entropically favourable so eqm lies to the right
what is the macrocyclic effect
complexes of macrocyclic ligands show even greater stability than chelated complexes
what are the metals that have anomalous trends in stability constants?
Cu(OH2)6 when replaced by 6NH3 ligands, K5 has sudden drop to a low value and K6 too small to measure
what is the usual trend in stability constants when 6H2O are replaced by 6NH3?
steadily decreasing because each intermediate has less H2O ligands to displace so less likely to be replaced
NH3 is bulkier and pushed electron density onto metal - less electrophilic so less likely to gain NH3
what reaction has a very unusual trend in K values? and why? (S)
6H2O being replaced with 4Br-
K decreased rapidly from K1 to K2, then slower from K2 to K3, increase from K3 to K4
different due to change in geometry, K4 more favourable due to entropy change
what reaction has a very unusual trend in K values? and why? (ss)
ligand substitution with ligands of different field strengths e.g. water to bipy, different due to change in spin state
what are the 4 factors that can affect stability constants in ligand substitution
1. size of ligand (steric hindrance/bulky)
2. electronegativity of ligand (EW or ED to make M more Nu-/El+)
3. change in geometry of complex
4. change in spin state of complex when ligands have different field strengths
what is a lewis acid
electron pair acceptor
what is a lewis base
electron pair donor
what determines the acidity of a hexa aqua complex?
charge density (oxidation state and size) of metal, polarises bond - protons more easily lost in solution to form partial/full OH- complex
do hard bases interact more strongly with soft or hard acids? what type of interaction is this?
hard acids
interactions are more electrostatic (+/- charges)
do soft bases interact more strongly with soft or hard acids? what type of interaction is this?
soft acids
interactions are more covalent (donor/acceptor orbitals)
define ambidentate ligands and give examples
ligands which have two possible centres of donation/attachement
e.g. SCN - S = soft, N = hard
SMe2O (DMSO), use O or S
what is a hard Lewis base?
small, low polarisability, high electronegativity
what is a hard Lewis acid?
small, large positive charges or high charge density
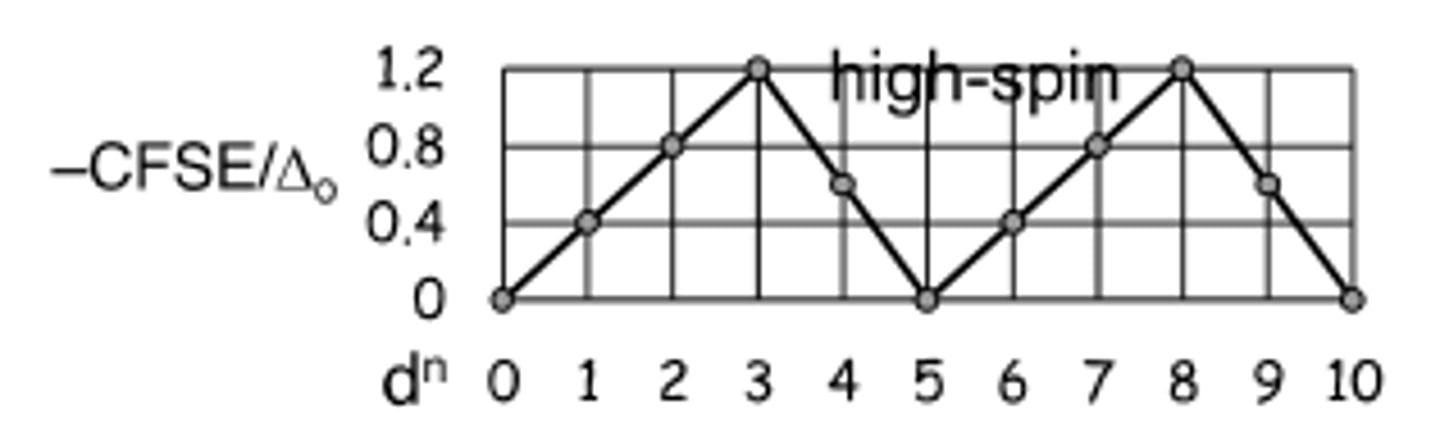
what is the Irving Williams series
trend in CFSE due to variation of the M(II) central ion on the stabilities of transition metal complexes
CFSE increase till d3, decrease to d5, increases until d8, decreases to d10 due to ionic radius
why are certain TM complexes certain colours?
equation: E = hv, c = vλ
the larger the energy (CFSE) the larger the frequency absorbed (shorter wavelength) so the smaller the frequency emitted and the longer the wavelength
what types of transition metals form linear complexes? why?
TMs with electron configuration of d10 e.g. Ag+
because CFSE has no effect on geometry so ligands can sit as far apart from one another like normal due to electron repulsion
how to calculate βn?
K1 x K2 x K3... x Kn = βn