Cell cycle regulators
Introduction
In the article on cell cycle checkpoints, we looked at the why of cell cycle transitions: the factors that a cell considers when deciding whether or not to move forward through the cell cycle. These include both external cues (like molecular signals) and internal cues (like DNA damage).
Cues like these act by changing the activity of core cell cycle regulators inside the cell. These core cell cycle regulators can cause key events, such as DNA replication or chromosome separation, to take place. They also make sure that cell cycle events take place in the right order and that one phase (such as G1_11start subscript, 1, end subscript) triggers the onset of the next phase (such as S).
In this article, we'll look at a few of the most important core cell cycle regulators: proteins called cyclins, enzymes called Cdks, and an enzyme complex called the APC/C.
Cyclins
Cyclins are among the most important core cell cycle regulators. Cyclins are a group of related proteins, and there are four basic types found in humans and most other eukaryotes: G1_11start subscript, 1, end subscript cyclins, G1_11start subscript, 1, end subscript/S cyclins, S cyclins, and M cyclins.
As the names suggest, each cyclin is associated with a particular phase, transition, or set of phases in the cell cycle and helps drive the events of that phase or period. For instance, M cyclin promotes the events of M phase, such as nuclear envelope breakdown and chromosome condensation1,2^{1,2}1,2start superscript, 1, comma, 2, end superscript.
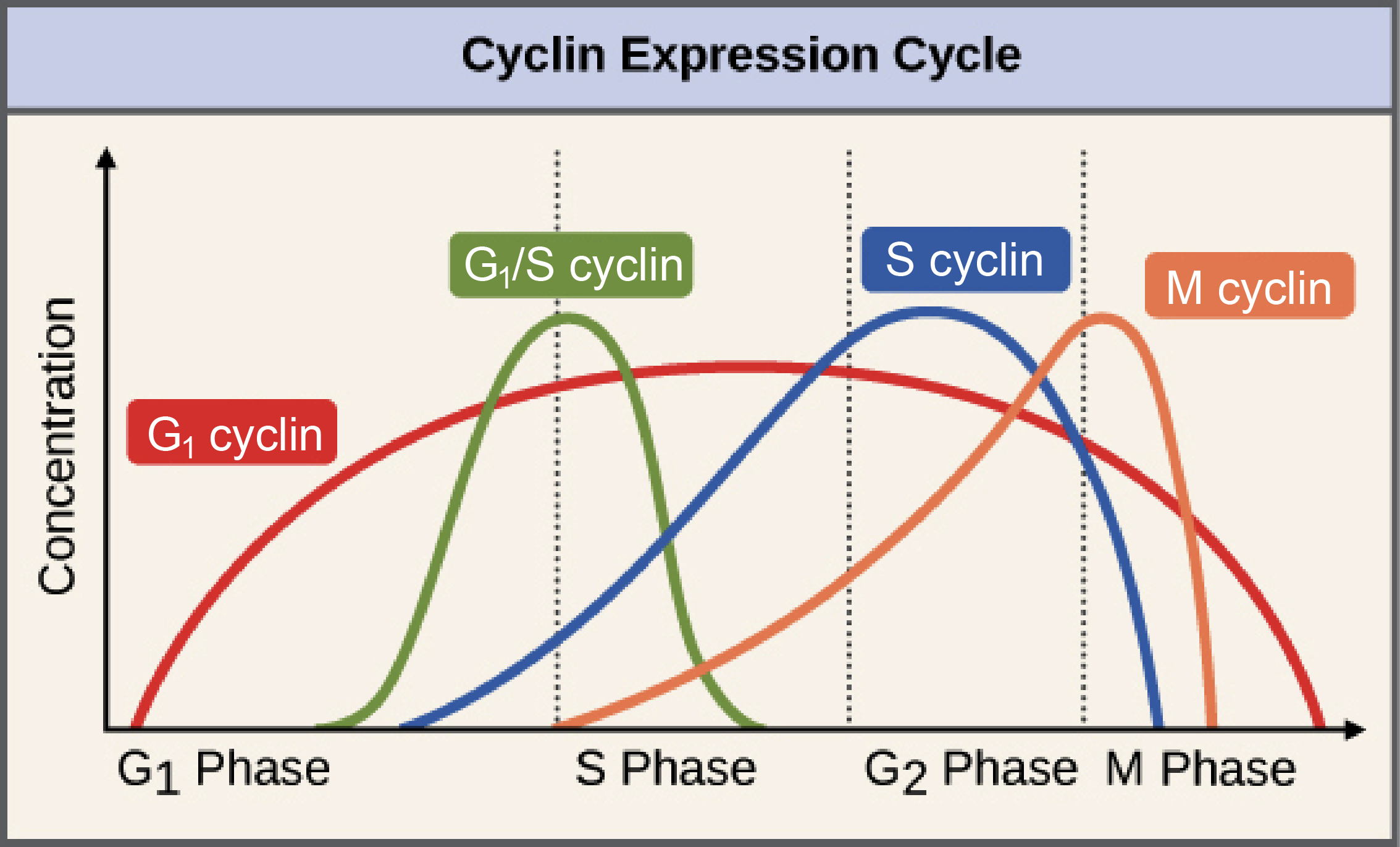
Diagram: the cyclin expression cycle. This is a graph showing how concentrations of the various cyclins change in a cell over the course of the cell cycle.
G1 cyclin: low in G1, rising slowly to a peak in mid-S phase, then dropping slowly back down to zero at the end of M phase.
G1/S cyclin: very low for most of the cell cycle, with a sharp, symmetrical peak at the G1/S transition.
S cyclin: low in early G1, rising slowly through late G1 and S, peaking in early G2 and dropping sharply back to zero in early M phase.
M cyclin: very low through all of G1, rising slowly through, peaking at the G2/M transition, and dropping sharply to zero in the middle of M phase.
Image modified from "Control of the cell cycle: Figure 2," by OpenStax College, Biology (CC BY 3.0). Modification of original work by WikiMaMa.
The levels of the different cyclins vary considerably across the cell cycle, as shown in the diagram at right. A typical cyclin is present at low levels for most of the cycle, but increases strongly at the stage where it's needed. M cyclin, for example, peaks dramatically at the transition from G2_22start subscript, 2, end subscript to M phase. G1_11start subscript, 1, end subscript cyclins are unusual in that they are needed for much of the cell cycle.
Cyclin-dependent kinases
In order to drive the cell cycle forward, a cyclin must activate or inactivate many target proteins inside of the cell. Cyclins drive the events of the cell cycle by partnering with a family of enzymes called the cyclin-dependent kinases (Cdks). A lone Cdk is inactive, but the binding of a cyclin activates it, making it a functional enzyme and allowing it to modify target proteins.
How does this work? Cdks are kinases, enzymes that phosphorylate (attach phosphate groups to) specific target proteins. The attached phosphate group acts like a switch, making the target protein more or less active. When a cyclin attaches to a Cdk, it has two important effects: it activates the Cdk as a kinase, but it also directs the Cdk to a specific set of target proteins, ones appropriate to the cell cycle period controlled by the cyclin. For instance, G1_11start subscript, 1, end subscript/S cyclins send Cdks to S phase targets (e.g., promoting DNA replication), while M cyclins send Cdks to M phase targets (e.g., making the nuclear membrane break down).
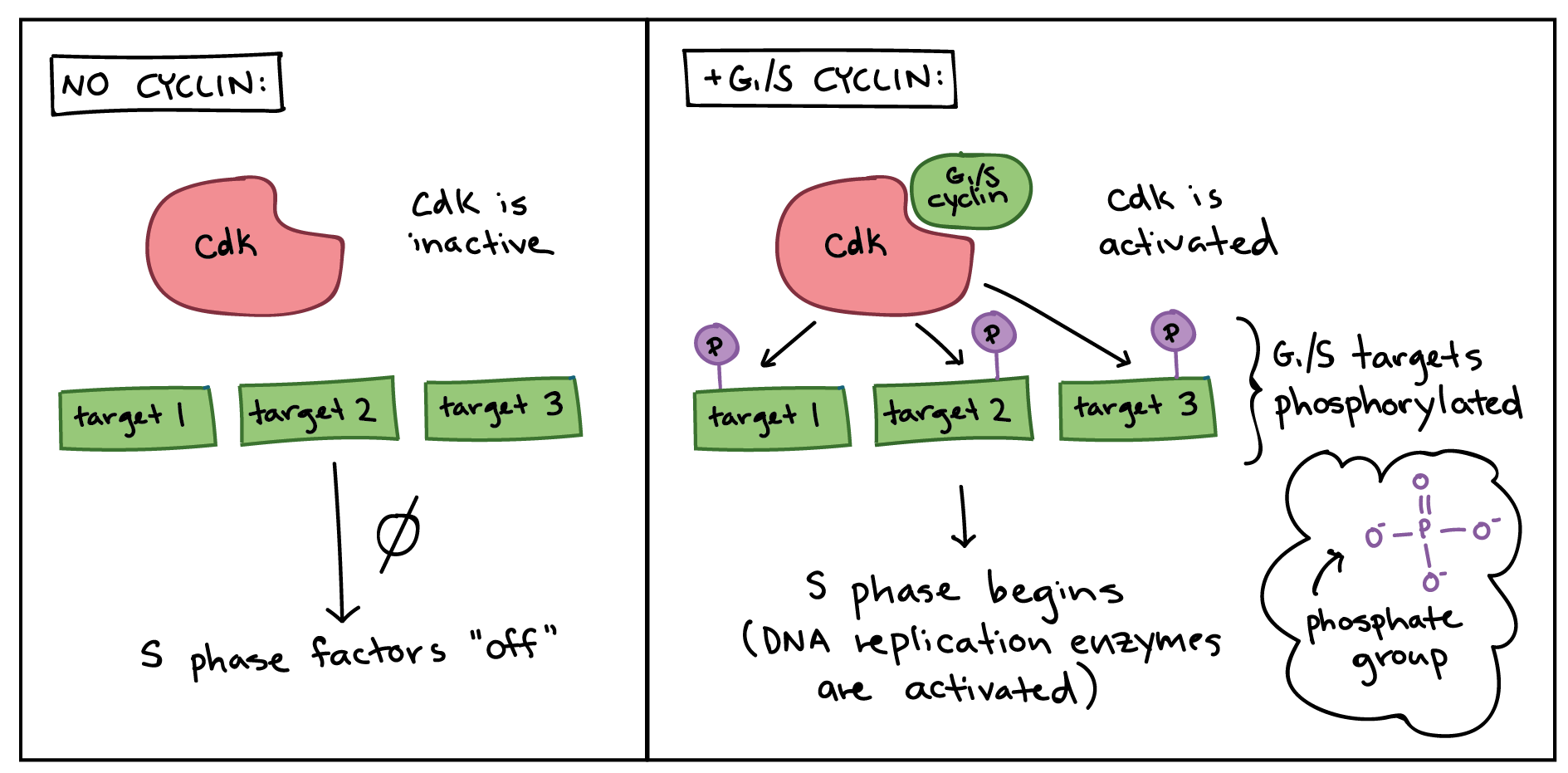
Simplified diagram showing how cyclins modify activity of Cdks.
Left panel (no cyclin): no cyclin is present, Cdk is inactive, and targets specific to the G1/S transition are not phosphorylated. Nothing happens, and S phase factors remain "off."
Right panel (+G1/S cyclin): the G1/S cyclin is present and binds to the Cdk. The Cdk is now active and phosphorylates various targets specific to the G1/S transition. The phosphorylated targets cause the activation of DNA replication enzymes, and S phase begins.
In general, Cdk levels remain relatively constant across the cell cycle, but Cdk activity and target proteins change as levels of the various cyclins rise and fall. In addition to needing a cyclin partner, Cdks must also be phosphorylated on a particular site in order to be active (not shown in the diagrams in this article), and may also be negatively regulated by phosphorylation of other sites3,4^{3,4}3,4start superscript, 3, comma, 4, end superscript.
Cyclins and Cdks are very evolutionarily conserved, meaning that they are found in many different types of species, from yeast to frogs to humans. The details of the system vary a little: for instance, yeast has just one Cdk, while humans and other mammals have multiple Cdks that are used at different stages of the cell cycle. (Yes, this kind of an exception to the "Cdks don't change in levels" rule!) But the basic principles are quite similar, so that Cdks and the different types of cyclins can be found in each species5^55start superscript, 5, end superscript.
Maturation-promoting factor (MPF)
A famous example of how cyclins and Cdks work together to control cell cycle transitions is that of maturation-promoting factor (MPF). The name dates back to the 1970s, when researchers found that cells in M phase contained an unknown factor that could force frog egg cells (stuck in G2_22start subscript, 2, end subscript phase) to enter M phase. This mystery molecule, called MPF, was discovered in the 1980s to be a Cdk bound to its M cyclin partner6^66start superscript, 6, end superscript.
MPF provides a good example of how cyclins and Cdks can work together to drive a cell cycle transition. Like a typical cyclin, M cyclin stays at low levels for much of the cell cycle, but builds up as the cell approaches the G2_22start subscript, 2, end subscript/M transition. As M cyclin accumulates, it binds to Cdks already present in the cell, forming complexes that are poised to trigger M phase. Once these complexes receive an additional signal (essentially, an all-clear confirming that the cell’s DNA is intact), they become active and set the events of M phase in motion7^{7}7start superscript, 7, end superscript.
The MPF complexes add phosphate tags to several different proteins in the nuclear envelope, resulting in its breakdown (a key event of early M phase), and also activate targets that promote chromosome condensation and other M phase events. The role of MPF in nuclear envelope breakdown is shown in simplified form in the diagram below.
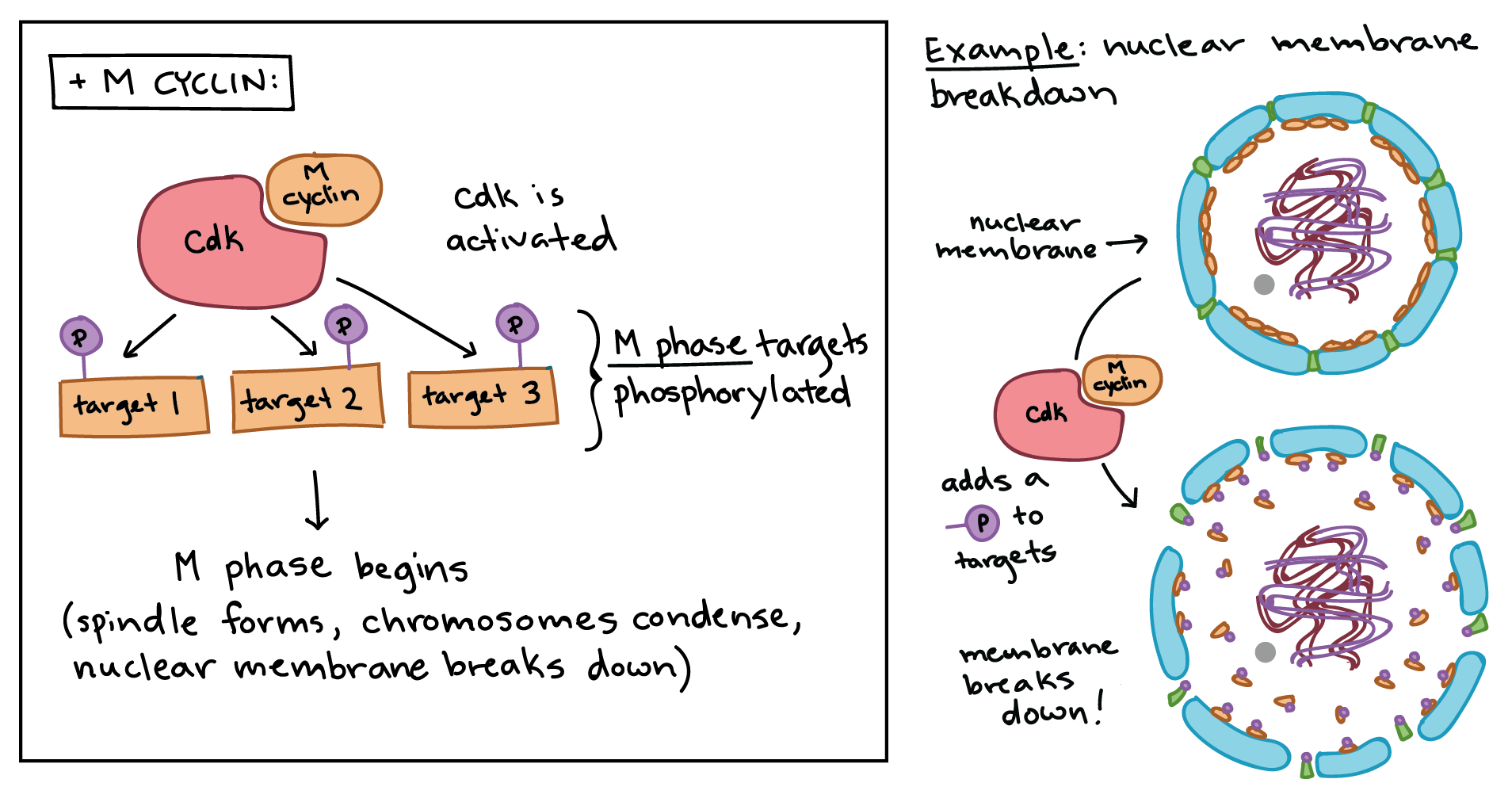
Simplified diagram showing how Cdk and M cyclin combine to form MPF.
Left panel: The MPF complex phosphorylates various targets specific to M phase, and the phosphorylated targets cause spindle formation, chromosome condensation, nuclear membrane breakdown, and other events of early M phase.
Right panel: Specific example of MPF triggering nuclear envelope breakdown. The MPF complex phosphorylates proteins in the nuclear envelope, resulting in the fragmentation of the nuclear membrane into vesicles (and release of some of the proteins from the membrane).
The anaphase-promoting complex/cyclosome (APC/C)
In addition to driving the events of M phase, MPF also triggers its own destruction by activating the anaphase-promoting complex/cyclosome (APC/C), a protein complex that causes M cyclins to be destroyed starting in anaphase. The destruction of M cyclins pushes the cell out of mitosis, allowing the new daughter cells to enter G1_11start subscript, 1, end subscript. The APC/C also causes destruction of the proteins that hold the sister chromatids together, allowing them to separate in anaphase and move to opposite poles of the cell.
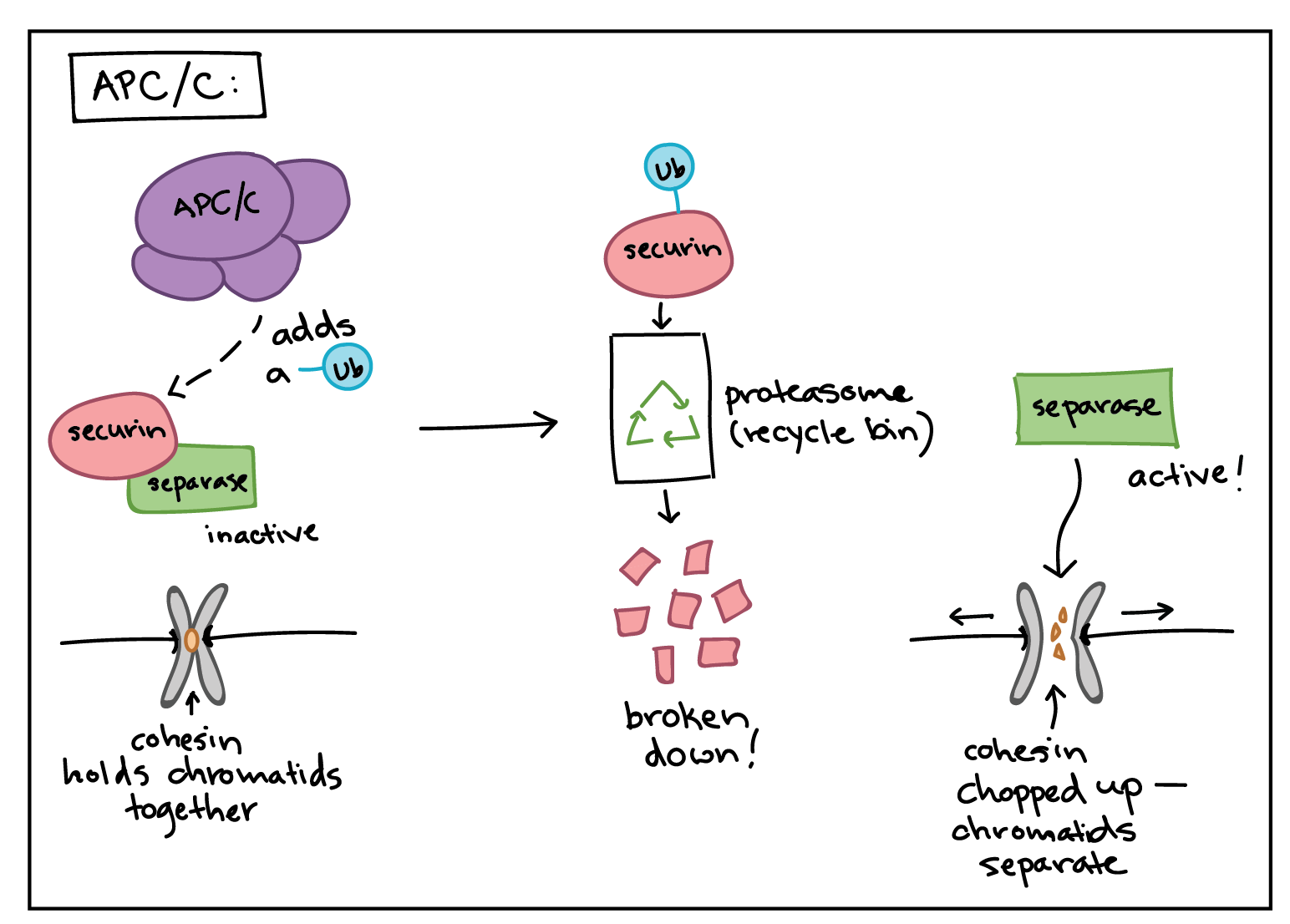
How does the APC/C do its job? Like a Cdk, the APC/C is an enzyme, but it has different type of function than a Cdk. Rather than attaching a phosphate group to its targets, it adds a small protein tag called ubiquitin (Ub). When a target is tagged with ubiquitin, it is sent to the proteasome, which can be thought of as the recycle bin of the cell, and destroyed. For example, the APC/C attaches a ubiquitin tag to M cyclins, causing them to be chopped up by the proteasome and allowing the newly forming daughter cells to enter G1_11start subscript, 1, end subscript phase8^{8}8start superscript, 8, end superscript.
The APC/C also uses ubiquitin tagging to trigger the separation of sister chromatids during mitosis. If the APC/C gets the right signals at metaphase, it sets off a chain of events that destroys cohesin, the protein glue that holds sister chromatids together8,9^{8,9}8,9start superscript, 8, comma, 9, end superscript.
The APC/C first adds a ubiquitin tag to a protein called securin, sending it for recycling. Securin normally binds to, and inactivates, a protein called separase.
When securin is sent for recycling, separase becomes active and can do its job. Separase chops up the cohesin that holds sister chromatids together, allowing them to separate.
Checkpoints and regulators
Cdks, cyclins, and the APC/C are direct regulators of cell cycle transitions, but they aren’t always in the driver’s seat. Instead, they respond to cues from inside and outside the cell. These cues influence activity of the core regulators to determine whether the cell moves forward in the cell cycle. Positive cues, like growth factors, typically increase activity of Cdks and cyclins, while negative ones, like DNA damage, typically decrease or block activity.
As an example, let's examine how DNA damage halts the cell cycle in G1_11start subscript, 1, end subscript. DNA damage can, and will, happen in many cells of the body during a person’s lifetime (for example, due to UV rays from the sun). Cells must be able to deal with this damage, fixing it if possible and preventing cell division if not. Key to the DNA damage response is a protein called p53, a famous tumor suppressor often described as “the guardian of the genome.” 10^{10}10start superscript, 10, end superscript
p53 works on multiple levels to ensure that cells do not pass on their damaged DNA through cell division3^{3}3cubed. First, it stops the cell cycle at the G1_11start subscript, 1, end subscript checkpoint by triggering production of Cdk inhibitor (CKI) proteins. The CKI proteins bind to Cdk-cyclin complexes and block their activity (see diagram below), buying time for DNA repair. p53's second job is to activate DNA repair enzymes. If DNA damage is not fixable, p53 will play its third and final role: triggering programmed cell death so damaged DNA is not passed on.
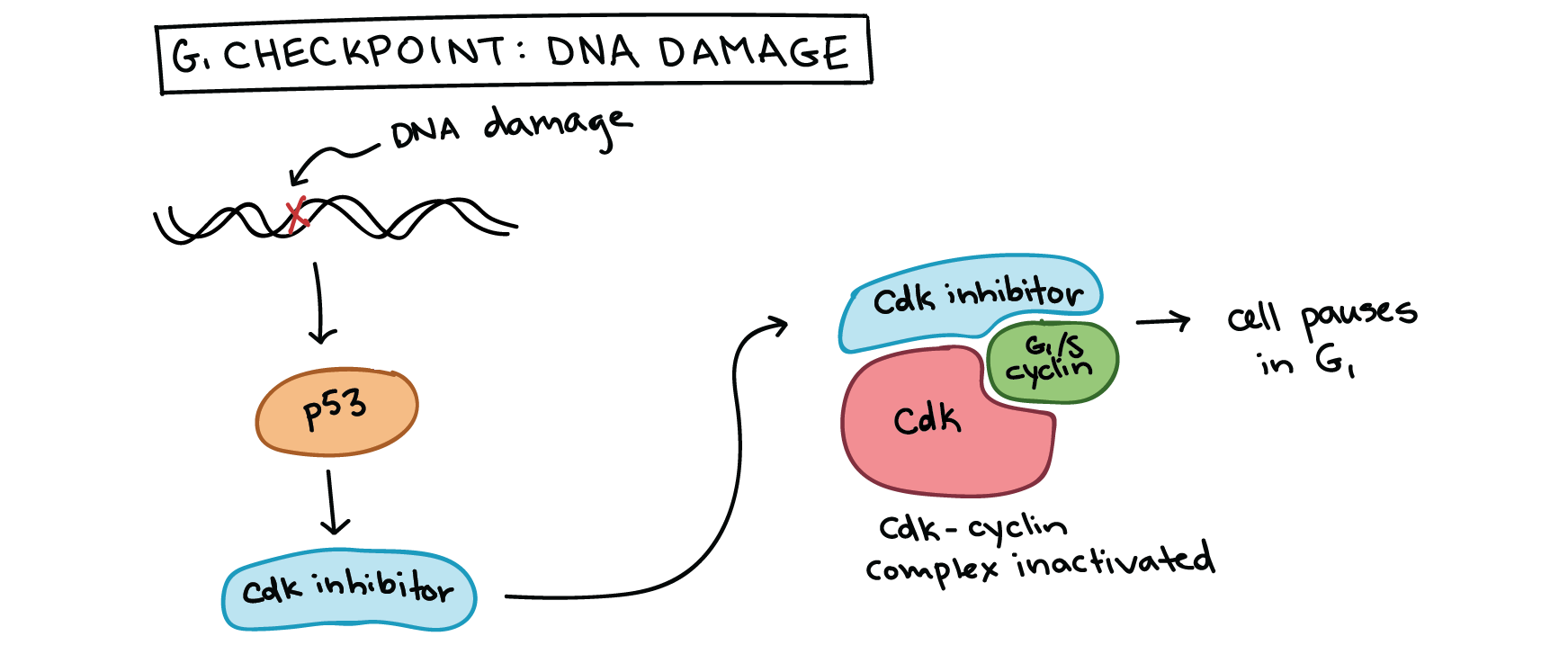
Simplified diagram of how p53 halts the cell cycle at the G1/S checkpoint. p53 is activated by DNA damage and causes production of a Cdk inhibitor, which binds to the Cdk-G1/S cyclin complex and inactivates it. This halts the cell in G1 and prevents it from entering S phase, allowing time for the DNA damage to be fixed.
By ensuring that cells don't divide when their DNA is damaged, p53 prevents mutations (changes in DNA) from being passed on to daughter cells. When p53 is defective or missing, mutations can accumulate quickly, potentially leading to cancer. Indeed, out of all the entire human genome, p53 is the single gene most often mutated in cancers.11^{11}11start superscript, 11, end superscript p53 and cell cycle regulation are key topics of study for researchers working on new treatments for cancer.
 Knowt
Knowt
